This is a product which has abundant nattokinase activity.
We focused on “nattokinase”, an enzyme derived from Bacillus subtilis natto, established a production method of “Fermented Soybean Extract” which contains a rich amount of nattokinase and launched the product before the rest of the world did. The stinky odour of natto as well as Bacillus subtilis natto and Vitamin K2 are removed. Moreover, the soybeans used for this material are non-GMO (non-Genetically Modified Organisms).
This product has gone down well with the Japanese market as well as the US, Korean, Taiwanese and Chinese markets ever since its launch. As a pioneer of nattokinase, we will meet every customer’s demand in terms of both technology and raw materials with the achievements we have accomplished.
- What is nattokinase?
-

An enzyme called nattokinase was discovered in natto, a Japanese traditional fermented food. It is produced during the fermentation process of soybeans by Bacillus subtilis natto and is a serine protease which consists of 275 amino acid residues and molecular weight 27,724.
Nattokinase enhances the thrombolytic ability and it has three primary functionalities: 1) to degrade fibrin directly, 2) to activate degradation promoting substances and 3) to inactivate degradation inhibiting substances which are produced by stress, etc.
How thrombus is generated and degraded
In human’s body, the reaction occurs as described below and thrombus is constantly generated and degraded. When the activity of plasmin, a thrombolytic enzyme which exists in one’s body, weakens, thrombus is more likely to be formed. Nattokinase, like the plasmin, is a fibrinolytic enzyme that directly degrades fibrin (*1). In addition, it has been proven that nattokinase promotes pro-urokinase to urokinase (*2) and helps tissue Plasminogen Activator (t-PA) increase by degrading Plasminogen Activator Inhibitor – 1 (PAI-1), by which eventually plasminogen is activated (*3).
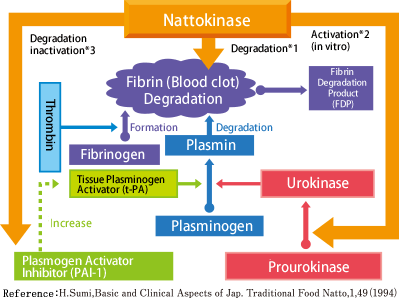
How thrombus is generated and degraded
- History of Fermented Soybean Extract
-
- 1995:
- Commencement of development of Fermented Soybean Extract
- 1998:
Establishment of production method of Fermented Soybean Extract containing an abundant amount of nattokinase
First manufacture and launching of Fermented Soybean Concentrated Extract (9,800FU/g) & Fermented Soybean Extract “NSK-FD” (13,000FU/g)
- 1999:
- First export of Fermented Soybean Extract NSK to Korean and Taiwanese pharmaceutical companies
- 2001:
- Introduction of “NSK-SD” (20,000FU/g) from which natto odour was removed
- 2003:
- Transferring of sole selling right of “Fermented Soybean Extract NSK-SD” in the US to a Japanese pharmaceutical company and its first export
- 2006:
-
JBSL obtains US patent as “platelet aggregation inhibitor & platelet aggregation inhibiting health food” (US7,014,851)
JBSL obtains process patent & substance patent on removal of vitamin K2
- Features
-
Vitamin K2 has been removed
People who take “warfarin” are normally told, by a doctor, to be restrained from eating food that contains a large amount of vitamin K2 such as natto, green/yellow vegetables and chlorella because such food weakens the effect of warfarin. However, we have developed a vitamin K2-removal technology and employed it for the production of Fermented Soybean Extract NSK so that even those people can take nattokinase.
Patents
![[Patent regarding vitamin K2 removal from
Fermented Soybean Extract NSK]/JP3,834,048 JP3,881,494](images/nsk/img_patent.gif)
This includes a process as well as concentrate, paste, powder, granule, capsules, drink and food in a tablet form of Bacillus subtilis natto culture medium which contains nattokinase but virtually no vitamin K2.
Differences between our product, competitors’products and counterfeits
Although nattokinase is the name of a single enzyme, many pseudo enzymes that are claimed as nattokinase are sold on the market. A peptidase produced by Bacillus subtilis in Japan, a protease by Bacillus subtilis in Taiwan and China, a cheonggukinase by analogous bacteria of Bacillus subtilis in Korea and a composite protease agent by Aspergillus oryzae in the US are sold as nattokinase. Some of them are similar to nattokinase but are still all distinct enzymes. These enzymes dissolve, more or less, in vitro artificial fibrin or that on a petri dish. Nevertheless, that does not necessarily mean that this reaction occurs in human’s body too and, also, the safety has been questioned.
We have established a simple nattokinase-differentiation method (IBox method) that distinguishes nattokinase from other enzymes with HPLC analysis of the decomposition style of oxidised insulin B-chain. With this method, we have made it possible to easily differentiate nattokinase from other pseudo-nattokinase enzymes.
- Efficacy
-
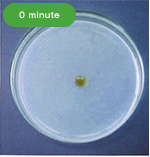
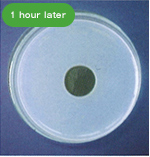
・Fibrinolytic Effect
The following pictures show the fibrinolytic effect of nattokinase (30μL (=60FU) of Fermented Soybean Extract 2,000 FU/mL) on artificial fibrin, created on a plate, over the course of time.
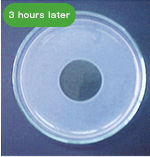
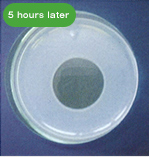
・Blood Flow Improving Effect
The volume of blood flow increased with the intake of NSK-SD and, thus, the blood flow improving effect was observed.
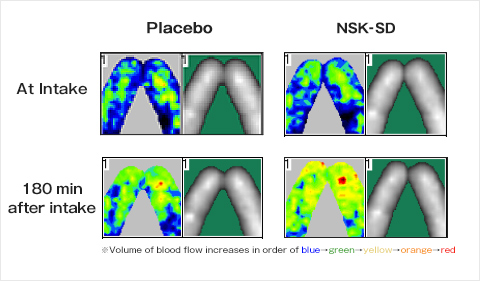
Changes in hemodynamics of middle fingers of both hands
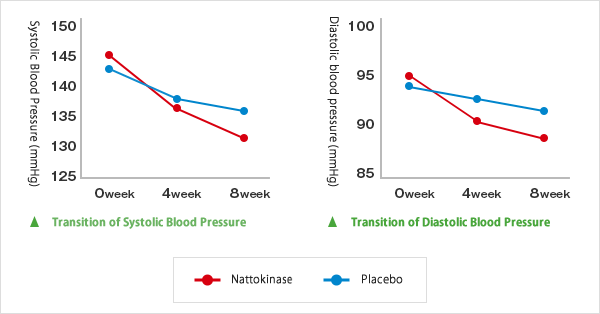
・Blood Pressure Reducing Effect
The reduction in systolic blood pressure and diastolic blood pressure after the intake of NSK-SD was observed.
・Improved Fibrinolysis Effect & Coagulation Inhibitory Effect
・Anti-platelet Effect
・Safety & Efficacy on Patients Taking Warfarin
- Safety & Stability
-
Safety of NSK-SD
Although natto has been eaten for a very long time in Japan, we do not solely rely on that fact and we endeavour to guarantee the safety.
Safety Studies
- 1.Single Oral Dose Toxicity Study
- 2.13-week Repeated Oral Dose Toxicity Study
- 3.Chromosomal Aberration Test
- 4.Ames Test
- 5.Pathogenicity Study of NSK-SD Producing Bacteria
- 6.Safety Study on Taking Natural Super Kinase-II (1-5: animal and microbial studies, 6-7:human clinical studies)
- 7.Safety Study on Concomitant Use of Warfarin and Nattokinase
The studies listed above have proven the safety of nattokinase.
Safety Management
The materials used for NSK are all non-GMO and non‐animal.
※The safety mentioned here only applies to our product, NSK-SD. Therefore this does not apply to nattokinase materials of other manufacturers.
The Safety and Biochemical Characteristics of NSK-SD
- pH Stability:
- pH 5.5-10
- Optimum pH:
- pH 10.5
- Thermostability:
- lower than 50℃
- Optimum temperature:
- 65℃
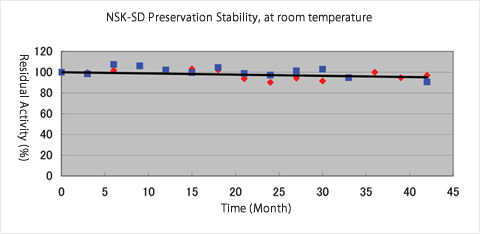
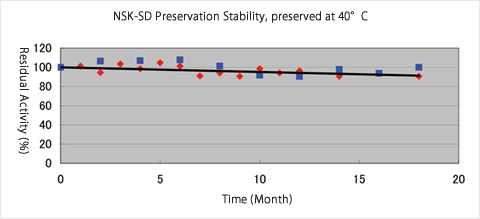
- Preservation Stability:
- table for 42 months at room temperature and 18 months at 40℃
We supply these technical data sheets.
- Product Specification
-
Fermented Soybean Extract Product Specification
Fermented Soybean ExtractNSK-SDProduct with high enzyme activity, with virtually no smell by our unique
technology
Spray-dried type
- Property:
- fine powder, white to milky white in colour with slight natto odour
- Specification:
- Nattokinase Activity: More than 20,000 FU/g
Vitamin K2: Less than 0.1 ppm
Recommended Intake
We recommend taking greater than or equal to 2,000 FU per day.
The nattokinase activity 2,000 FU equals approximately 100 mg of NSK-SD.Japan Health and Nutrition Food Association (JHFA)Standard
JHFA has set criteria of the recommended daily intake of “fermented soybean extract food” as follows.
”Fermented soybean extract processed food”・・・≧ 2,000 FU as nattokinase
”Fermented soybean extract containing food”・・・1,000 FU ≦, < 2,000 FU as nattokinaseJapan Health and Nutrition Food Association ( JHFA ) Standard
Japan NattoKinase Association (JNKA) Standard
The products which meet the following four criteria are considered to meet JNKA’s standard and, thus, are allowed to put the JNKA mark.
- 1.Contain Bacillus subtilis natto products
- 2.Contain ≧ 2,000 FU/day of nattokinase
- 3.Indicate the nattokinase content with FU
- 4.The safety is proved
“FU” stands for Fibrinolytic Unit which is a unit that indicates nattokinase activity. We have suggested and enlightened a nattokinase activity measuring method (fibrin degrading method) using fibrin as its framework. It has been employed for the standard judgement of JHFA and JNKA and “FU” has become well-known as the unit that indicates nattokinase activity.
- Application & Blending Examples
-
Primary Application
Compatibility of NSK-SD and other materials
You can refer to our data sheets on compatibility evaluation of NSK-SD and other materials (food and health food ingredients).
Examples of materials that do not hinder nattokinase activity of NSK-SD
Ashitaba Chalcone Powder
Ginkgo Leaf Extract
Coenzyme Q10, etc※As to evaluations of naturally derived materials, we recommend testing each material individually because the results differ, depending on manufacturers, producing areas, harvest seasons (Lot), and so forth. Please feel free to contact us regarding this matter.











